Boxed Warning Risk Calculator
Every year, the FDA updates its list of boxed warnings for prescription drugs - and these changes aren’t just bureaucratic footnotes. They directly affect who gets treated, how they’re monitored, and sometimes, whether they live or die. If you’ve ever seen a thick black border around a warning on a medication label, you’ve seen one of the most powerful tools in modern drug safety. These aren’t generic cautions. They’re the FDA’s strongest signal: boxed warnings mean real, preventable, life-threatening risks.
What Exactly Is a Boxed Warning?
A boxed warning, also called a black box warning, is the highest safety alert the FDA can require for a prescription drug. It’s not buried in fine print. It’s placed at the very top of the drug’s prescribing information, enclosed in a thick black border that takes up at least half the page width. The FDA has used this format since the 1970s, but its power comes from what’s inside: specific, evidence-based dangers that have been confirmed through real-world use, not just lab studies.These warnings don’t say “may cause side effects.” They say: “This drug can kill you if used incorrectly.” For example, fentanyl patches carry a boxed warning that they’re deadly for opioid-naive patients - meaning someone who’s never taken opioids before. Valproic acid can cause fatal liver damage, but only if you don’t check liver enzymes in the first six months. These aren’t guesses. They’re facts drawn from thousands of patient reports in the FDA’s Adverse Event Reporting System (FAERS).
In 2022, over 400 drugs in the U.S. had active boxed warnings - about 12% of all prescription medications. That number is rising. In 2022 alone, the FDA added or revised 47 boxed warnings, a 12% jump from the year before. The biggest drivers? Immunomodulators, cancer drugs, and newer diabetes and weight-loss medications like GLP-1 agonists.
What Changed in the Latest Updates?
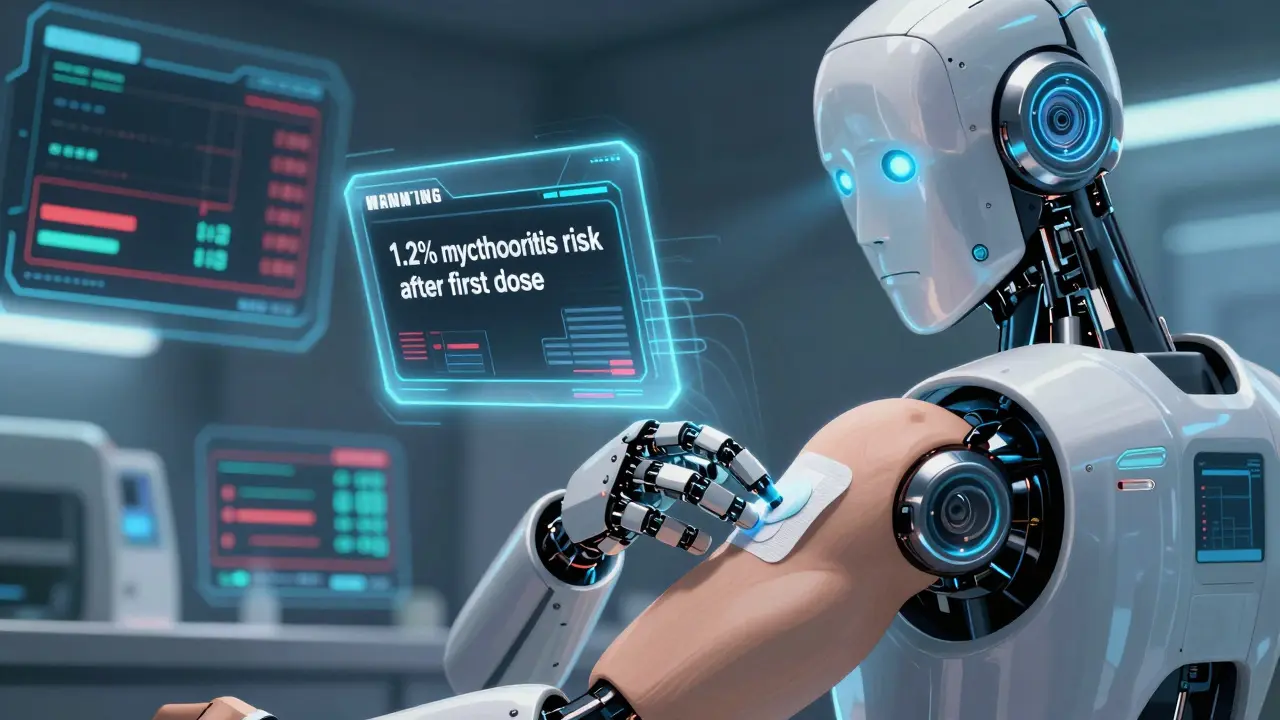
The 2023-2024 updates brought some of the most significant shifts in decades. For the first time, the FDA started requiring quantified risk data in every new boxed warning. No more vague phrases like “risk of liver injury.” Now, warnings must say: “1.2% of patients under 30 developed myocarditis after the first dose.” That’s a game-changer.One major update came for immune checkpoint inhibitors like pembrolizumab and nivolumab. New data from the FDA’s Sentinel Initiative - which tracks 200 million patient records - showed a clear pattern: autoimmune colitis and myocarditis were occurring more frequently than previously known, especially in younger patients. The updated warning now includes specific monitoring timelines: “Check troponin and CK within 72 hours of first symptoms of chest pain or shortness of breath.”
For GLP-1 agonists - drugs like semaglutide and tirzepatide - the FDA added a new boxed warning about severe gastrointestinal complications. Earlier reports linked these drugs to gastroparesis and bowel obstruction, but now the warning includes data: “1 in 250 patients treated for over 12 months experienced gastric outlet obstruction requiring hospitalization.” That’s not theoretical. That’s real-world evidence from electronic health records.
Even older drugs got updates. Warfarin’s warning now explicitly says: “Do not initiate in patients with INR > 5.0 without urgent reversal.” That’s new. Previously, it just said “risk of bleeding.” Now, it tells you exactly when to stop.
Why These Changes Matter to Patients and Doctors
These aren’t just paperwork changes. They change how doctors prescribe and how patients take their meds.Take clozapine, a drug used for treatment-resistant schizophrenia. It carries a boxed warning for agranulocytosis - a drop in white blood cells that can be fatal. Since the 1990s, patients have needed weekly blood tests. But now, the updated warning requires labs every two weeks after the first six months - a practical adjustment based on long-term data showing risk drops sharply after that point. That means fewer clinic visits, less cost, and better adherence.
On the flip side, some updates make things harder. A new warning for the antibiotic moxifloxacin now says: “Avoid in patients with known QT prolongation or those taking other QT-prolonging drugs.” That’s a problem in emergency rooms, where doctors need to treat pneumonia fast. A 2022 Medscape survey found 44% of physicians felt boxed warnings sometimes delay critical care. But the FDA’s point is: if you skip the check, you might kill someone.
For patients, clearer warnings mean better understanding. A 2021 FDA patient forum showed that 78% of people taking isotretinoin (Accutane) for acne followed the iPledge program - which prevents pregnancy during treatment - because the warning explained exactly what could happen. “I didn’t know it could cause birth defects until they showed me the numbers,” said one participant. That’s the goal: clarity, not fear.
How Health Systems Are Adapting
Hospitals and pharmacies aren’t just reading the updates - they’re building systems around them.The Joint Commission now requires all accredited hospitals to have triple-check protocols for drugs with boxed warnings. At Henry Mayo Newhall Hospital, pharmacists must verify opioid tolerance before dispensing fentanyl patches. At University of Michigan, nurses complete a 2.7-hour training module before they can administer methotrexate - because giving it daily instead of weekly can be fatal.
Electronic health records (EHRs) are getting smarter too. The FDA’s 2022 pilot program tested “dynamic” boxed warnings that adjust based on patient data. If a patient has kidney disease, the system highlights the warning for drugs cleared by the kidneys. If they’re over 65, it flags fall-risk drugs. Early results showed a 37% drop in alert fatigue - meaning clinicians actually pay attention now.
But problems remain. A Reddit thread from January 2023 with 142,000 pharmacy members found that 61% of warfarin overrides happened because the EHR didn’t show the patient’s last INR result. If the system doesn’t give you the full picture, even the clearest warning won’t help.
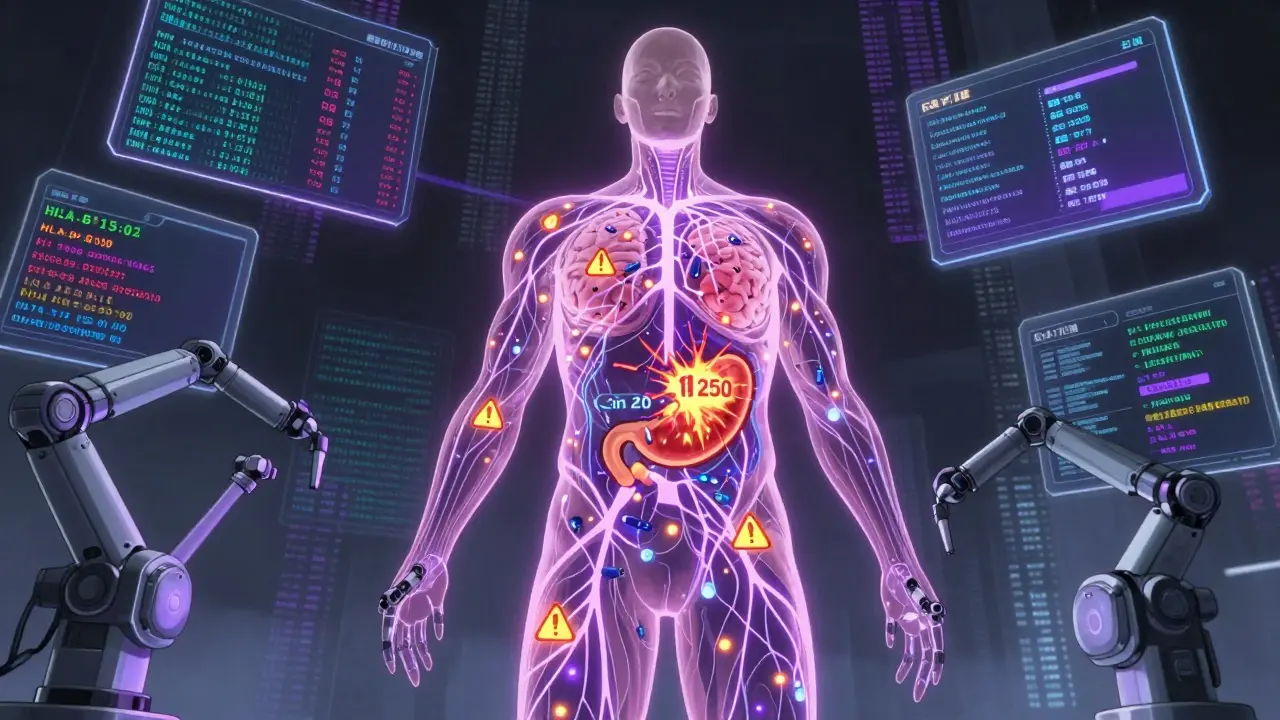
The Debate: Are Boxed Warnings Working?
There’s no consensus among experts.Dr. Thomas J. Moore from Johns Hopkins found that only 43% of boxed warnings include specific, actionable steps. If a warning says “monitor for liver damage,” but doesn’t say how often or with what test, doctors just ignore it. That’s why the 2023 requirement for quantified data is so important.
But the FDA counters: since 2015, boxed warnings have prevented an estimated 12,000 serious adverse events each year. That’s lives saved.
Prescribing patterns tell another story. After rosiglitazone got its cardiovascular warning in 2007, prescriptions dropped 70%. But pioglitazone, with a nearly identical warning, saw almost no drop - because it didn’t get the same media attention. That’s the problem with warnings: they’re only as strong as the awareness behind them.
And then there’s the economic side. Drugs with new boxed warnings lose an average of 22% in revenue within a year. But drugs like warfarin - which has no real alternative - keep selling. So the market doesn’t always react. What matters is whether the warning changes behavior, not just sales.
What’s Next?
The FDA’s 2023-2027 plan calls for 25% more boxed warnings based on real-world data - not just clinical trials. That means more updates for drugs used long-term: antidepressants, statins, diabetes meds, and painkillers.Expect more warnings tied to genetic risk factors. For example, HLA-B*15:02 testing before carbamazepine use is already standard in some Asian populations to prevent Stevens-Johnson syndrome. That’s likely to become universal.
And the push for dynamic alerts will expand. Imagine a warning that pops up only if your patient is on five or more drugs, or if they’re over 70. That’s not science fiction - it’s coming.
One thing’s clear: the era of vague, generic warnings is over. The new standard is precision. Specific risks. Clear monitoring steps. Real numbers. And that’s better for everyone - patients, doctors, and pharmacists alike.
What is the difference between a boxed warning and a regular warning?
A regular warning is a general caution, often listed in the side effects section. A boxed warning is the FDA’s strongest alert - it’s required when there’s clear evidence of life-threatening or preventable harm. It’s placed at the top of the prescribing information with a thick black border and must include specific risks, at-risk populations, and monitoring requirements.
Can a drug be taken if it has a boxed warning?
Yes, many drugs with boxed warnings are still essential and widely used. The warning doesn’t mean the drug is banned - it means it must be used carefully. For example, warfarin carries a boxed warning for major bleeding, but it’s still the best option for many patients with atrial fibrillation. The key is following the monitoring and contraindications exactly.
Why do some drugs get boxed warnings years after they’re approved?
Clinical trials involve thousands of patients, but real-world use involves millions. Rare or long-term side effects often only show up after years of use. The FDA uses post-marketing surveillance systems like FAERS and the Sentinel Initiative to detect these risks. When patterns emerge - like a spike in heart inflammation after a new drug is widely prescribed - the FDA acts.
Do boxed warnings affect drug prices?
Yes, but indirectly. Drugs with new boxed warnings often see a 22% drop in sales within a year, which can reduce revenue for manufacturers. However, if the drug has no alternatives - like insulin or warfarin - sales stay stable. The price itself rarely changes, but insurers may add prior authorization requirements, making access harder.
How do I know if my medication has a boxed warning?
Check the medication guide that comes with your prescription - it’s required by law. You can also ask your pharmacist or look up the drug on the FDA’s website under “Drug Safety Communications.” If you’re using an electronic health record, the system should flag the warning when the prescription is entered. Never assume a drug is safe just because it’s been on the market for years - warnings are updated regularly.

Sazzy De
Been seeing more of these black boxes lately and honestly it’s a relief. Used to be like playing Russian roulette with prescriptions. Now at least you know what you’re getting into.
Blair Kelly
Let’s be real - the FDA’s been way too slow on this. They waited YEARS for data on GLP-1 agonists while people were getting hospitalized. This isn’t progress - it’s damage control. And don’t even get me started on how EHRs still don’t show the last INR. That’s malpractice waiting to happen.
Carolyn Whitehead
It’s kinda beautiful how they’re finally using real-world data instead of just lab numbers. Makes me trust the system more even if it’s scary to see how many drugs can actually kill you if you’re not careful.
Kimberly Reker
I work in a clinic and we just updated our protocol for checkpoint inhibitors last month. Now we have a checklist: troponin at 24h, CK at 48h, and if the patient says ‘my chest feels weird’ - we don’t wait. We act. It’s a small change but it’s saved two people already. These warnings aren’t red tape - they’re lifelines.
April Allen
The quantification shift is monumental. Saying ‘1.2% of patients under 30 developed myocarditis’ instead of ‘risk of myocarditis’ transforms clinical decision-making. It allows for risk stratification, shared decision-making, and informed consent that’s actually informed. This is evidence-based medicine finally catching up to epidemiology.
Shubham Dixit
Look, in India we don’t even get these warnings properly. Pharmacies hand out warfarin like candy. No INR checks. No monitoring. No training. The FDA’s doing its job, but here? We’re still treating prescriptions like over-the-counter snacks. Until we fix that, all these black boxes are just pretty letters on a page for us. You can’t have precision medicine in a system that doesn’t even track who got the pill.
And don’t get me started on how doctors still prescribe clozapine without checking ANC. It’s not ignorance - it’s negligence. And no, the government won’t fix it. We have to demand it.
Every time someone dies because they didn’t know about the warning, it’s not an accident. It’s a failure of the system. And if you think this is just about drugs, you’re wrong. It’s about who gets protected and who gets left behind.
My uncle took metformin for 12 years. Never had a check-up. Got lactic acidosis. Died in a hospital with no one knowing he was on it. That’s the real cost of vague warnings. Not lawsuits. Not revenue drops. Lives.
We need mandatory training for prescribers. Not just in the US. Globally. And we need to make the warnings loud, visual, and unavoidable. Not buried in a PDF. Not in fine print. On the bottle. On the receipt. On the pharmacy counter. In the language people actually speak.
The FDA’s doing better. But they’re not the only ones who need to change.
Rohit Kumar
There’s a deeper truth here. Boxed warnings aren’t just about safety - they’re about power. Who gets to decide what’s dangerous? Who gets to decide what’s worth the risk? The FDA says ‘this kills’ - but the market says ‘but people want it.’ And the patient? They’re just trying to survive. This isn’t regulation. It’s a negotiation between life, profit, and bureaucracy.
Think about it. Warfarin’s been around since 1954. It’s cheap. It’s effective. But it kills. So we monitor it obsessively. But insulin? Also deadly if misused. No boxed warning. Why? Because it’s essential. Because we can’t afford to scare people away. So we accept the risk. That’s not science. That’s moral calculus.
And now we’re adding genetic markers? HLA-B*15:02? That’s not medicine. That’s anthropology. We’re starting to treat people not as patients but as data points with ancestry. And that’s beautiful - and terrifying.
Medicine used to be about trust. Now it’s about algorithms and thresholds. We’re trading intuition for statistics. And I’m not sure we’re better off.
Sheila Garfield
My mum’s on rivaroxaban now. She’s 78. The pharmacist showed her the warning - printed it out, read it to her, even drew a little diagram. She understood it. That’s the win. Not the data. Not the stats. That moment. When someone actually gets it.
Kelly Weinhold
Just want to say thank you to everyone who works on this - the researchers, the pharmacists, the EHR developers. I know it’s not sexy. No one’s posting TikToks about troponin levels. But you’re literally saving lives one alert at a time. And I see you. Keep going.
Eliana Botelho
Wait - so you’re telling me the FDA now requires numbers in warnings? That’s hilarious. Last year they added a warning for metformin saying ‘risk of vitamin B12 deficiency’ - but didn’t say how much or how often. So now they’re demanding numbers but still leaving the easy stuff vague? This isn’t precision. It’s performative.
And why is no one talking about the fact that most of these updates come after lawsuits? The FDA doesn’t act because it’s right - they act because they’re scared of being sued. That’s not safety. That’s liability management.
Also - why do only the expensive drugs get the fancy new warnings? Metformin? No box. But semaglutide? Full black border with graphs? Coincidence? I think not.
Adarsh Uttral
they changed warfarin warning to say dont start if inr >5? wow. took me 2 mins to google that. why didnt they just say that 10 years ago? i mean come on
Niamh Trihy
The real test isn’t whether the warning exists - it’s whether the system can support it. I’ve seen EHRs flag a boxed warning, then the clinician clicks ‘ignore’ because the next screen asks for a reason - and there’s no option for ‘I know what I’m doing.’ That’s design failure. Not user error.
And we need to stop treating patients like passive recipients. If the warning says ‘check troponin within 72 hours,’ why not send them a text reminder? Why not link it to their portal? Why not make it part of their care plan - not just a pop-up?
This isn’t just about the FDA. It’s about how we design care. The warning is the beginning - not the end.
Sidhanth SY
As someone who grew up in a household where medicine was never questioned - I’m glad we’re finally asking harder questions. My dad took clozapine for 15 years. He never knew he needed weekly blood tests until his 3rd hospitalization. That’s not just a warning - that’s a system failure. And now? We’re fixing it. Slowly. But we’re fixing it.
Jodi Olson
It’s interesting how the most life-saving tools are also the most bureaucratic. We need a warning that says ‘this can kill you’ - but then we need a thousand forms to prove we read it. The irony isn’t lost on me.
Katie and Nathan Milburn
Just a note: the 37% drop in alert fatigue isn’t because the warnings are better - it’s because they’re now contextual. That’s the real breakthrough. Not the content. The delivery. Tailored alerts are the future. Generic alerts are noise.
Kelly Weinhold
And to the person who said the FDA only acts after lawsuits - you’re right. But maybe that’s how change happens. Sometimes you need a tragedy to wake people up. And now we’re waking up. Slowly. But we’re waking up.