Generic drugs make up over 90% of all prescriptions filled in the U.S. But if you’ve ever been told your usual medication isn’t available, you know how fragile that system really is. These aren’t rare glitches-they’re systemic failures. Between 2018 and 2023, the FDA recorded more drug shortages than in any previous decade. And the vast majority? Generic drugs. Not brand-name ones. Not new innovations. The cheap, everyday medicines millions rely on for blood pressure, infections, cancer treatment, and anesthesia.
Why Do Generic Drugs Keep Running Out?
The short answer: manufacturing problems. According to the FDA, nearly 62% of all drug shortages since 2020 stem directly from issues at production facilities. That’s not a coincidence. It’s the result of a supply chain built on thin margins and zero room for error.
Imagine a factory that makes a simple antibiotic. The equipment is old. The cleaning protocols are tight. One tiny contamination-bacteria in a pipe, a faulty filter-forces the entire line to shut down. Not for a day. Not for a week. Sometimes for months. While the facility fixes the problem, the FDA has to inspect it again. No approval, no production. Meanwhile, hospitals are scrambling. Patients are delayed. Some end up on less effective, more expensive alternatives.
The Global Manufacturing Trap
Most of the active ingredients in generic drugs-called APIs-are made in just two countries: China and India. About 80% of global API production happens there. That’s not because they’re the best. It’s because they’re the cheapest.
But that convenience comes with risk. A single flood in a Chinese chemical plant, a labor strike in an Indian facility, or even a new environmental regulation can ripple across the entire U.S. drug supply. There’s no backup. No redundancy. If one plant goes down, and it’s the only one making that specific API, the drug vanishes from shelves nationwide.
And it’s not just the ingredients. Finished dosage forms-pills, injections, capsules-are also heavily outsourced. Half of all U.S. drug manufacturing facilities are overseas. That means shipping delays, customs holdups, and geopolitical tensions all become drug shortage risks.
No Safety Net: The Zero-Inventory Model
Unlike car makers who keep spare parts on hand, generic drug manufacturers operate on a just-in-time model. They make exactly what’s needed, when it’s needed. No extra stock. No buffer. Why? Because profit margins are razor-thin.
A branded drug might earn a 30-40% profit. A generic? Often less than 15%. With prices driven down by pharmacy benefit managers (PBMs) and intense competition, manufacturers can’t afford to store extra inventory. They can’t afford to upgrade equipment. They can’t afford to hire extra staff. So when something breaks-whether it’s a machine, a power outage, or a regulatory violation-they don’t have the resources to fix it fast.
And when one manufacturer quits? That’s it. No one else steps in. Why? Because the market doesn’t pay enough. If a drug sells for $2 a bottle and costs $1.80 to make, there’s no incentive to invest in a new production line. Even if thousands of patients need it.
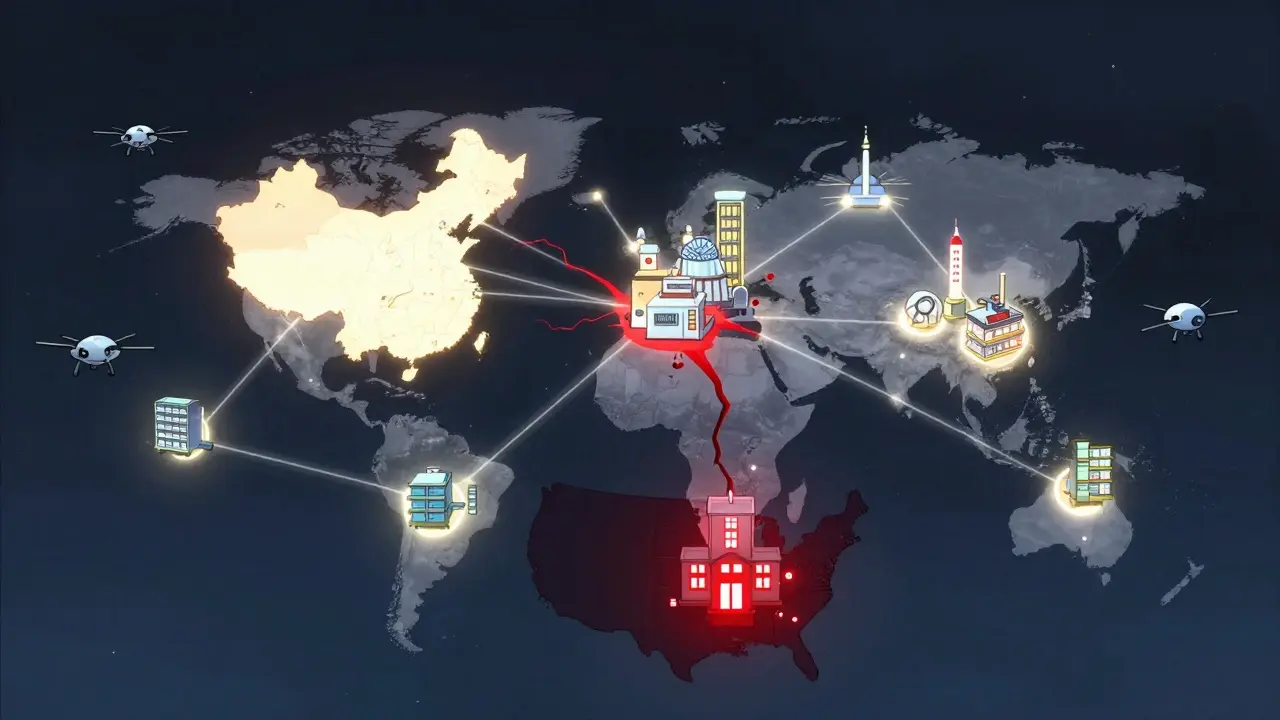
Single Points of Failure
One in five drug shortages involves a product made by only one company. That’s called sole-sourcing. And it’s a disaster waiting to happen.
Take a common chemotherapy drug. Only two factories in the world make it. One gets shut down for quality violations. The other can’t ramp up fast enough. The result? Cancer patients go without treatment. Hospitals ration doses. Doctors have to choose who gets the medicine and who doesn’t.
This isn’t rare. It’s standard. The consolidation of manufacturers over the last decade has meant fewer companies making more drugs. That sounds efficient. But it’s actually dangerous. One plant failure can knock out dozens of life-saving medications at once.
Who’s Driving the Prices Down?
It’s not just manufacturers. It’s the middlemen. Three pharmacy benefit managers-CVS Caremark, Express Scripts, and OptumRx-control about 85% of prescription drug spending in the U.S. They negotiate prices with manufacturers. They decide which drugs go on insurance formularies. And they push for the lowest possible price.
That sounds good for consumers. But it backfires. When manufacturers can’t make a profit on a generic drug, they stop making it. Or they make less of it. Or they cut corners on quality. The result? Shortages. And when shortages happen, PBMs don’t always help. Some even push hospitals to use drugs that are already in short supply, simply because they’re cheaper to buy-ignoring the fact that those drugs are running out.
The Federal Trade Commission called this practice ‘opaque’ and ‘unaccountable.’ No one knows how these decisions are made. And patients pay the price.
The Domino Effect
It’s not just one problem. It’s a chain reaction.
- Low profit margins → manufacturers exit the market
- Fewer manufacturers → less competition → less innovation in production
- Consolidation → single points of failure
- Overseas production → vulnerability to global shocks
- No inventory buffer → no recovery time
- PBM pricing pressure → even lower margins
Each step makes the next one worse. And when a crisis hits-like a pandemic, a natural disaster, or a regulatory crackdown-the whole system trembles.
What’s Being Done?
There are efforts to fix this. The RAPID Reserve Act, introduced in 2023, proposes creating a national stockpile of critical generic drugs-not just for bioterrorism, like the current Strategic National Stockpile, but for everyday shortages. It also offers tax incentives to bring API production back to the U.S.
The FDA is trying to speed up inspections and approvals. Some hospitals are building their own emergency reserves. The AMA has started urging insurers to stop pushing drugs that are already in short supply.
But none of this fixes the root problem: the economy of generic drugs doesn’t reward reliability. It rewards the lowest bid.
What This Means for Patients
If you take a generic drug-whether it’s metformin for diabetes, levothyroxine for thyroid issues, or ciprofloxacin for an infection-you’re already in the crosshairs of this system. You might not notice the shortage until your pharmacy calls and says, ‘We don’t have it.’ Then you wait. Or you switch to a different brand. Or you pay more. Or you skip doses.
For cancer patients, anesthetics, or antibiotics, these delays aren’t inconveniences. They’re life-or-death. One study found that nearly a quarter of all shortage reports don’t even explain why the drug is gone. No cause. No timeline. Just silence.
Pharmacists now spend 50-75% more time managing shortages than they did 10 years ago. That’s time not spent counseling patients, checking interactions, or ensuring safety.
The Bottom Line
Generic drug shortages aren’t accidents. They’re engineered by a system that prioritizes cost over care. We’ve outsourced production, eliminated competition, cut margins to the bone, and removed any safety net. And now we’re surprised when things break.
Fixing this won’t mean more regulations or more inspections. It means changing the rules of the game. Paying manufacturers enough to make drugs reliably. Rewarding redundancy, not just low prices. Building domestic capacity. And treating essential medicines like the public health infrastructure they are-not just commodities to be bid down to the lowest bidder.
Until then, the next shortage isn’t a question of if. It’s a question of when.

Neela Sharma
This isn't just about drugs-it's about how we value life in a market that only counts dollars. We built a system where a cancer patient's survival hinges on the cheapest bid. Imagine if your heart medication was treated like a commodity on Alibaba. We don't need more inspections. We need a moral reset.
Sarah Little
The PBM oligopoly is structurally defective. Their fee-for-service model incentivizes volume over viability, creating negative externalities in supply chain resilience. Regulatory arbitrage in API sourcing exacerbates systemic fragility.
innocent massawe
I'm from Nigeria. We know what it means when medicines disappear. Here in the US, people act like it's a surprise. But in places where the system was never strong, we just learn to adapt. 😔
Tru Vista
PBMs are the real villains. They negotiate down prices so hard that no one can profit. Then they blame manufacturers. Total BS. Also typo: 'anesthetics' not 'anethetics'.
Philip Leth
I've had my levothyroxine pulled twice. No explanation. Just 'out of stock.' Meanwhile, my insurance still charges me $10 for a drug that doesn't exist. This is a scam.
Angela Goree
China and India are stealing our medicine supply! We used to make 70% of our drugs here-now it's 20%! This is national security failure! We need tariffs! We need bans! We need to bring back American factories-NOW! And stop letting foreign labs cut corners!!
Angela Fisher
This is all part of the Great Reset. The WHO and Big Pharma are working with the FDA to create controlled shortages so they can push vaccines and biologics. They want you dependent on expensive new drugs. That's why generics keep disappearing. Look at the funding sources. Look at the FDA inspectors who used to work for Pfizer. Coincidence? I think not. 🤫💉
Shruti Badhwar
The structural flaws outlined here are undeniable. However, policy interventions must be calibrated to avoid unintended consequences. A national stockpile, while commendable, may incentivize complacency among manufacturers unless paired with tiered reimbursement models that reward reliability over price. Domestic re-shoring requires subsidies-but these must be transparent and performance-based. Without accountability, we risk repeating the same failures with taxpayer money.